Development and design of continuous mammalian cell culture processes.
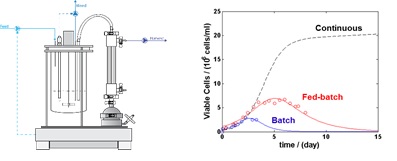
Therapeutic proteins, particularly monoclonal antibodies (mAbs) are the fastest growing type of biopharmaceuticals aiming towards the development of personalized treatment of cancer and various immunologic diseases. The increased production demand, number of therapeutics and the need for enhanced product quality, requires the development of new mammalian cell culture processes. In particular, perfusion cell cultures are a promising alternative, given their increased volumetric productivity and ability of stable operation at high viable cell density during months. In contrast to traditional fed-batch processes, the combination of constant cellular environment and the short product residence time reduces product heterogeneity.
The definition of suitable operating parameters is essential not to limit cellular growth and productivity. Aiming at the integration to a continuous downstream train, process control, robustness and automation are critical requirements of the perfusion culture.
Contact Person: Daniel Karst
