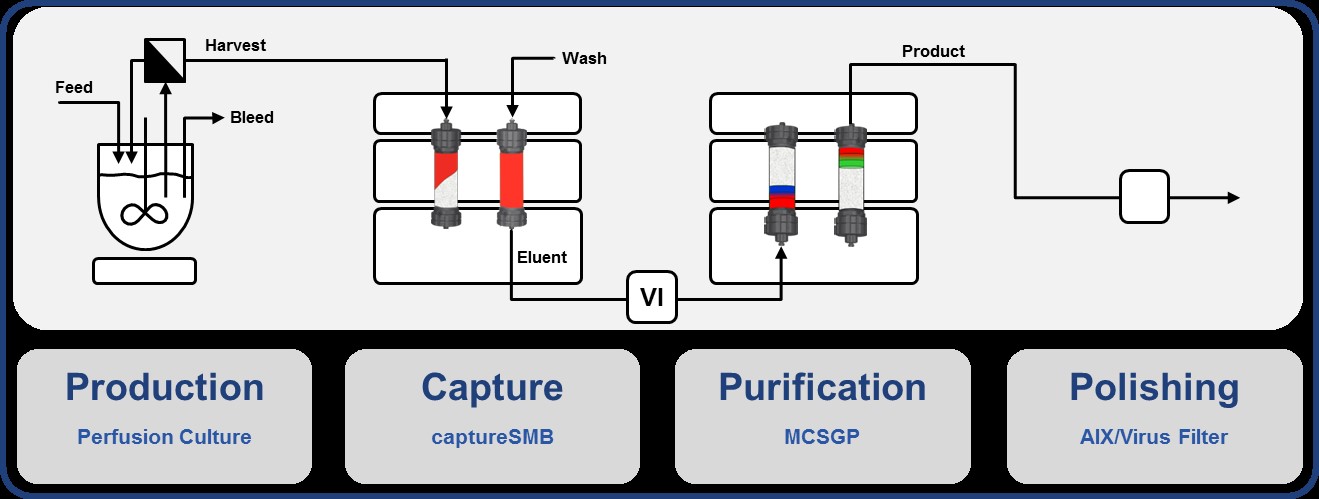
Integrated Continuous Biomanufacturing
Given their high complexity, recombinant therapeutic proteins, such as monoclonal antibodies (mAb), are expressed in mammalian cells. Processes in the biopharmaceutical industry typically rely on a series of batch-wise operations. Time and location of production, separation and polishing of the protein product are segregated. However, recent improvements of continuous up- and downstream processes suggest their final integration to a single process stream. Besides general benefits of continuous manufacturing, such as reduced equipment size, enhanced cost efficiency and high volumetric productivity, the steady state operation favors the consistency of product quality. The integration of single unit operations reduces the number of non-productive filtration and storage steps, thus leading to shorter processing times.
In this project we investigate the impact of continuous integrated operation on process performance and product quality. Consistent operation at enhanced product quality of desired therapeutic proteins by precise overall control and optimization is aimed at.
Contact Person: Prof. Dr. Massimo Morbidelli
Contact Person: Moritz Wolf
Contact Person: Sebastian Vogg