Carbon Interpenerated TiO2-based Anode Material for Lithium-ion Batteries
Rechargeable Lithium ion batteries (LIBs) have represented a class of the most promising alternative energy storage technology among various electrochemical storage devices. Compared with the traditional energy storages, LIBs have more advantages in term of high efficiency, high power density and environmental benignity. Therefore, LIBs play an important role in the applications of various portable electronic devices, large scale energy storages for electric vehicles and renewable energy such as solar power.1-3 Current LIBs have several issues to be addressed including cost, durability and safety problems. The key to improve LIB performance is to fabricate functional electrode materials. Titania (TiO2) is an excellent candidate as anode material for LIBs, competing with commercial graphite, due to its high abundance, low cost, high Li-insertion potential, structural and chemical stability, safety and environment friendliness.4-5 However, TiO2 has intrinsic drawbacks in practical application in LIBs, among which the low diffusivity of Li and poor electrical conductivity are two dominant issues. To advance TiO2 anode performance, there are two aspects generally acknowledged:
(1) Fabrication of TiO2 based nanostructures with specific morphology. The electrochemical properties of electrodes mainly depend on the structure, morphology and size of the active material. In particular, the nanoscale TiO2 has large active surface area which benefits high contact with the electrolyte; accordingly, the diffusion pathway for Li+ ions is effectively shortened, leading to higher capacity, faster charge/discharge rates and longer cycling life, with respect to conventional bulk TiO2.6-8
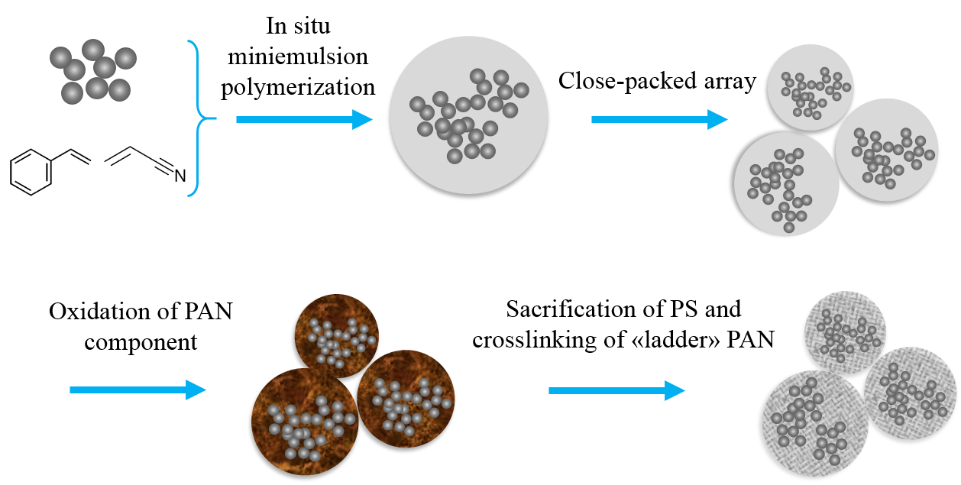
(2) Using TiO2-based hybrid materials as the active materials. As a semi-conducting material, TiO2 itself is facing significant challenge in terms of low electrical conductivity (~10-12 S m-1), resulting in low effective power density. Therefore, various TiO2-based hybrid composite materials have been explored to enhance the conductivity. Conductive coatings such as graphitic carbon are commonly used to improve electron transport. Moreover, it is worth mentioning that nanoparticles tend easily to aggregate either during their synthesis or post treating or galvanostatic cycling, leading to loss of surface area for Li+ ion transfer. A proper solution is to generate TiO2 nanoclusters, where the primary TiO2 nanoparticles are separated by conductive material at a certain distance. To achieve this aim, we propose to build a hierarchical structure for the TiO2-based anode material, starting from small primary active anatase TiO2 which will then be encapsulated and grouped to fractal, forming interconnected network meanwhile possesses micropores.
In this project, we aim to develop an architectural materials based on carbon-coated TiO2. The design is started from core-shell hybrid nanospheres, which have TiO2 nanoclusters as incorporated core and polymer as both host matrix and shell. These obtained monodispersed nanospheres will form close-packed array either through gravitational sedimentation or centrifugation. Through pyrolysis of the well-defined polymeric shell which contains the source of carbon, a graphene-like layer is generated and serves as both conductive material and mechanical supporter of the nanospheres after the pyrolysis, while the pyrolysis of the inner polymeric matrix will result in a conductive carbon network for both separating the TiO2 nanoparticles and conductivity. Therefore, the whole array will become well conductive.
Contact Person: Lu Jin
References:
(1) Marom, R.; Amalraj, S. F.; Leifer, N.; Jacob, D.; Aurbach, D. A review of advanced and practical lithium battery materials. Journal of Materials Chemistry 2011, 21 (27), 9938-9954.
(2) Kim, T. H.; Park, J. S.; Chang, S. K.; Choi, S.; Ryu, J. H.; Song, H. K. The Current Move of Lithium Ion Batteries Towards the Next Phase. Advanced Energy Materials 2012, 2 (7), 860-872.
(3) Goodenough, J. B.; Park, K.-S. The Li-Ion Rechargeable Battery: A Perspective. Journal of the American Chemical Society 2013, 135 (4), 1167-1176.
(4) Wagemaker, M.; Mulder, F. M. Properties and Promises of Nanosized Insertion Materials for Li-Ion Batteries. Accounts of Chemical Research 2012, 46 (5), 1206-1215.
(5) Chen, Z.; Belharouak, I.; Sun, Y. K.; Amine, K. Titanium-Based Anode Materials for Safe Lithium-Ion Batteries. Advanced Functional Materials 2013, 23 (8), 959-969.
(6) Hwang, H.; Kim, H.; Cho, J. MoS2 Nanoplates Consisting of Disordered Graphene-like Layers for High Rate Lithium Battery Anode Materials. Nano Letters 2011, 11 (11), 4826-4830.
(7) Moretti, A.; Kim, G.-T.; Bresser, D.; Renger, K.; Paillard, E.; Marassi, R.; Winter, M.; Passerini, S. Investigation of different binding agents for nanocrystalline anatase TiO2 anodes and its application in a novel, green lithium-ion battery. Journal of Power Sources 2013, 221 (0), 419-426.
(8) Ren, Y.; Liu, Z.; Pourpoint, F.; Armstrong, A. R.; Grey, C. P.; Bruce, P. G. Nanoparticulate TiO2(B): An Anode for Lithium-Ion Batteries. Angewandte Chemie International Edition 2012, 51 (9), 2164-2167